Week 13: Cooperative jeopardy
|
Today was our final class for the semester. We played Review Jeopardy. I really like how I changed it up this time so I thought I would share. If you are wanting to replicate this class, here are the jeopardy questions I used for free on our TPT page. All of the questions are from the handouts from the past 12 weeks of class.
Instead of having two teams duke it out, everyone had to work together. I called this Cooperative Jeopardy. Each student was able to choose a question they wanted from the board. If they answered correctly, they would earn a piece of candy for the class bowl. Depending on how many questions they answered correctly, determined how many pieces of candy the class received. I maintained the use of categories and signified level of difficulty with 100 questions, 200 questions, 300 questions, 400 questions and 500 questions. Because of this, they also earned better candy for the harder 500 questions. For
Even if the student didn't know any of the answers they would still get to pick a question and if they couldn't answer it, I would answer it correctly and leave the question on the board for someone else to grab. This allowed the question to be asked repeatedly until someone remembered the answer. The repetition was golden. I just want to say that the Lord was helping me today with this. The students earned exactly 42 pieces of candy and I had 14 students in attendance today. This divided evenly into 3 pieces of candy per student. I couldn't have planned that if I tried. If that had not worked out so perfectly, I probably would have asked several students to accept just two pieces and then reward them for their sacrifice with a choice of candy when everyone had their pieces. This demonstrates a willingness to place other's happiness before their own which is a character trait I want to encourage and reward. It is of the same heart as Jesus. |
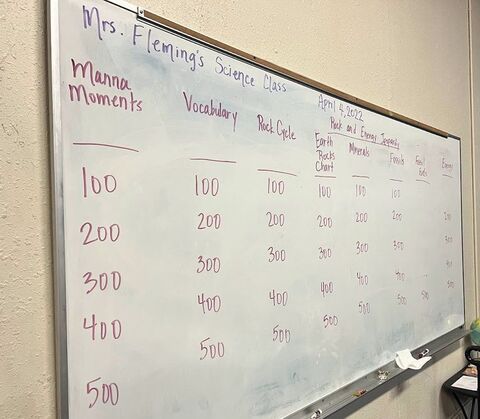
Here is what I placed on the board. It helped to have the candy lined up on the board so the students could visualize their class reward.
This was a fun class to teach. I love how the chemistry flowed into food science and then into nutrition.
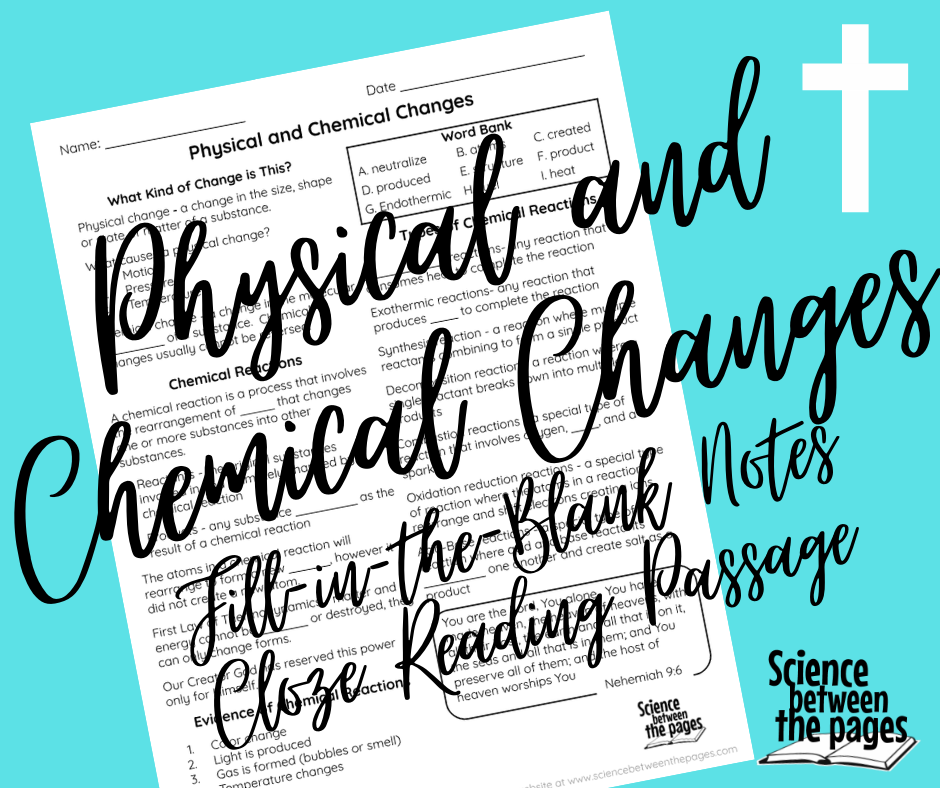
Week 12 Physical and Chemical changes
|
We are bringing it home.
The scripture that we began this class adventure with was Nehemiah 9:6 and today we will finish our unit up with this same scripture. It states, "You are the Lord, You alone. You have made heaven, the heaven of heavens, with all their host, the earth and all that is on it, the seas and all that is in them; and You preserve all of them; and the host of heaven worships You" The First Law of Thermodynamics states that matter and energy cannot be created or destroyed, they can only change forms. The power to create or destroy matter and energy is reserved solely for our Almighty God. Praise Him! I do not think that it is a coincidence that there are laws that govern the natural world, laws of physics etc. and the 10 commandments or the Law as given to the Israelites in the Bible of which also apply to us as Believers. God has an order to how to best respond to Him, treat each other, and order to the physical nature of this world. He is a magnificent Creator God. So when we look at a chemical reaction we are not witnessing the creation of new atoms, we are witnessing the rearrangement of atoms that consequently result in a substance change. |
How Do we know it is a chemical reaction?
The four evidences of chemical reactions that we touched on today through our note taking guide are:
1. Color Change
2. Light is produced
3. Gas in formed (bubbles or smell)
4. Temperature changes
1. Color Change
2. Light is produced
3. Gas in formed (bubbles or smell)
4. Temperature changes
Types of chemical change
In our notes we discovered:
My focus for this class was primarily on the endothermic and exothermic reactions. Endothermic reactions consume heat to complete the reaction making the product cooler to the touch. Exothermic reactions produce heat to complete the reaction making the product hotter to the touch.
- Endothermic reactions
- Exothermic reactions
- Synthesis reactions
- Decomposition reactions
- Combustion reactions
- Oxidation Reduction reactions
- Acid-Base reactions
My focus for this class was primarily on the endothermic and exothermic reactions. Endothermic reactions consume heat to complete the reaction making the product cooler to the touch. Exothermic reactions produce heat to complete the reaction making the product hotter to the touch.
Putting it to practice
The experiment that we conducted today in class is very simple yet perfect for the students to be able to conduct on their own and make their own conclusions. Here is the handout with the directions on how to do the experiment as well a place to place your data.
The goal of this experiment was to see which substances, when combined, would result in a rearrangement of atoms or chemical reaction.
We set up 6 different cups labeled
After the students placed all their ingredients in their respective cups, they then added drops of water or vinegar depending on the cup.
Out of all these options, there was only one chemical reaction and that was between the baking soda and vinegar.
The goal of this experiment was to see which substances, when combined, would result in a rearrangement of atoms or chemical reaction.
We set up 6 different cups labeled
- Sugar and Water
- Salt and Water
- Baking Soda and Water
- Sugar and Vinegar
- Salt and Vinegar
- Baking Soda and Vinegar
After the students placed all their ingredients in their respective cups, they then added drops of water or vinegar depending on the cup.
Out of all these options, there was only one chemical reaction and that was between the baking soda and vinegar.
The bubbler

After we discovered that baking soda and vinegar make a chemical reaction which can be seen through gas production, I had to use it!.
I followed Janice VanCleave's Bubbler experiment to use the carbon dioxide produced to make bubbles!
I followed Janice VanCleave's Bubbler experiment to use the carbon dioxide produced to make bubbles!
Review
Next week will be a huge review for us so we had a little warm up today with our DynoCards
Week 11 Vitamins and Minerals
|
John 15:26- "But when the Helper comes, whom I will send to you from the Father, the Spirit of truth, who proceeds from the Father, he will bear witness about me."
Vitamins and Minerals are our body's helpers. We have another Helper that helps us too, the Holy Spirit. The Holy Spirit helps us to absorb the nutrients in the Word of God just as Vitamin D helps us absorb the Calcium needed for our bones. Learning about how vitamins help absorption of nutrients and accelerate chemical processes in our bodies are just some of the objectives that we covered using the Vitamins and Minerals Handout. We also learned about fat and water soluble vitamins, which vitamins we need to replenish often and others not quite so often. There are brief descriptions of Vitamin A, B, C, D, and K as well as what minerals are and which are the major ones we need in our bodies. |
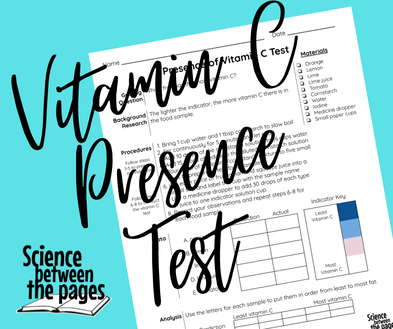
Testing for the presence of Vitamin C
When air meets up with fruit, the oxygen begins to break down the cell wall of fruit thus turning the fruit brown. This process is called oxidation. Vitamin C is an antioxidant and several fruits have it. I wanted to test to see what juice would would help slow the oxidation process of a freshly cut apple. In order to find this out, we have to test several juices for the presence of vitamin C.
We tested freshly squeezed
Whichever one we found to have the most vitamin C, I would apply to one half of my apple.
We tested freshly squeezed
- oranges,
- lemons,
- limes,
- store-bought lime juice, and
- tomatoes.
Whichever one we found to have the most vitamin C, I would apply to one half of my apple.
Before class, I prepared an indicator. This indicator contains cornstarch, water and iodine.
First, I measured a cup of water into a saucepan and added one Tablespoon of cornstarch to it. Stirring continually, I slowly boiled these two ingredients together for 5 minutes. After 5 minutes, I removed it from heat to let it cool.
Once it had cooled, I added 10 drops to 1/3 cup of water. My cornstarch solution had a gravy-like consistency. After I stirred this together, I added 15 drops of 1% iodine solution and stirred. The result was a dark blue indicator liquid.
First, I measured a cup of water into a saucepan and added one Tablespoon of cornstarch to it. Stirring continually, I slowly boiled these two ingredients together for 5 minutes. After 5 minutes, I removed it from heat to let it cool.
Once it had cooled, I added 10 drops to 1/3 cup of water. My cornstarch solution had a gravy-like consistency. After I stirred this together, I added 15 drops of 1% iodine solution and stirred. The result was a dark blue indicator liquid.

I had a student label some small cups with
While she was labeling the cups, the other students were making their predictions about which juices they thought had vitamin C and out of those which had the least to the most vitamin C present.
- orange,
- lemon,
- lime
- store bought lime juice
- tomato
While she was labeling the cups, the other students were making their predictions about which juices they thought had vitamin C and out of those which had the least to the most vitamin C present.
Freshly squeezed orange juice was the first juice that we tested.
We followed this procedure for the next four juices. I had some great help with squeezing and dropping!
- I placed 2 tsp of indicator liquid into the small cup labeled 'orange'
- I then squeezed a small mandarin orange in my juice squeezer (not sure the proper name for this)
- We then added 30 drops of the juice to the indicator liquid to see what would happen.
We followed this procedure for the next four juices. I had some great help with squeezing and dropping!
Here are our test results. We have store bought lime juice on the far left with the least amount of vitamin C. Next to that moving to the right are lime, lemon, tomato and orange. We had a slight accident with the tomato juice which is why there is less juice in there but before it spilled it didn't look as light as the orange juice.
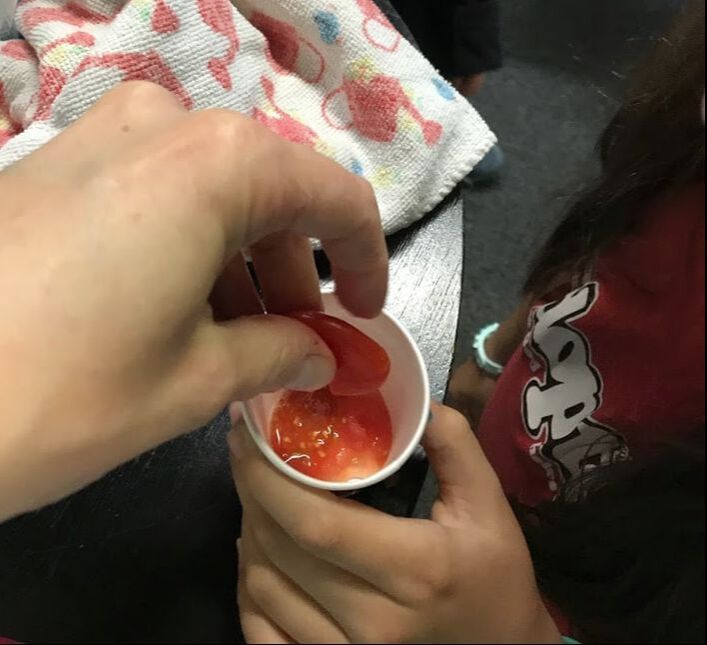
We decided to add orange juice to our apple and here are the results of that.
We decided to add orange juice to our apple and here are the results of that.
The apple half on the left has the added orange juice on it. It turns out that the vitamin C in the orange was adequate in reducing the oxidation process but do you want to eat an orange tasting apple??? Hmmm Food science problems.
Review of Physical Properties
We are wrapping up the semester of science and so reviews happen every week. Today I decided to review the physical properties of matter. I was so proud that the students were able to match most all the cards without much of a problem. I used Dynocards.
Week 10 Proteins and Fats

At the beginning of class, I read a devotion from How Great Is Our God by Louie Giglio called "That's Impossible." This devotion spoke about how God made living things with some essential building blocks called peptides. Each of these peptides have chemical components called amino acids and these amino acids make up proteins which is what part of our lesson in class was on today.
Psalm 146:6 "He is the Maker of heaven and earth, the sea, and everything in them"
Psalm 146:6 "He is the Maker of heaven and earth, the sea, and everything in them"

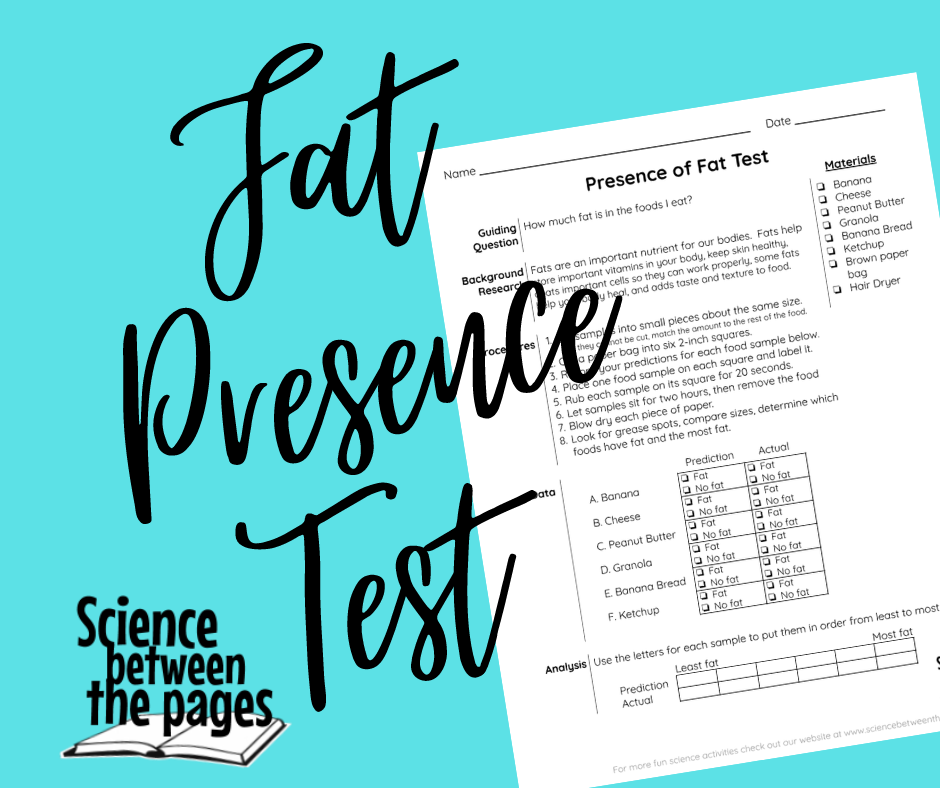
I jumped ahead a bit and started the Presence of Fat experiment because it was going to need some time to complete. For best results, you would want your food that you are testing for fat sit for 2 hours. Our food samples only sat for 45 minutes. It was an adequate amount of time.
I had done the experiment myself this past weekend and brought in my results to show them as well and compare. Another variable to what I did in class, is that I had four different students rubbing the food on the brown paper squares. When it is just one person, typically one person will apply similar pressure in their rub. It seems that some of my students were very gentle while others were determined.
I grabbed this experiment idea from Apologia's Human Physiology Textbook. This text is so thorough, I have really enjoyed using this textbook as a resource in my science classes. I went ahead and made an investigation sheet where you can put all your data and analysis on a simple sheet of paper.
I had done the experiment myself this past weekend and brought in my results to show them as well and compare. Another variable to what I did in class, is that I had four different students rubbing the food on the brown paper squares. When it is just one person, typically one person will apply similar pressure in their rub. It seems that some of my students were very gentle while others were determined.
I grabbed this experiment idea from Apologia's Human Physiology Textbook. This text is so thorough, I have really enjoyed using this textbook as a resource in my science classes. I went ahead and made an investigation sheet where you can put all your data and analysis on a simple sheet of paper.
|
The supplies you will need for this experiment are simple:
Before you begin the actual experiment, there is a place on the investigation sheet where you can make your predictions of whether you think each food has fat in it. Then you can also predict which foods have the most fat and the least and everything in between. I had one clever student today remember the nutrition facts on ketchup when we were learning how to read food labels. She shared with the class that ketchup did not have any fat. So clever! After predictions were made, I set to work placing the food on the brown paper bag squares and labeling them. I asked for volunteers on who would like to rub a food on the paper for 20 seconds. At my mark, they started rubbing. After the rub, I let the food sit on the paper until the end of class where we checked our results. |

Before the rub

After the rub

After two hours and a blow dry.
|
After we set that experiment up, we delved into our note taking guides on proteins and fats.
One of the things we learned is that proteins are not stored in our body like carbohydrates. We need to replenish our protein daily. On our note taking sheet, I placed a formula that will help them figure out how much protein they should be consuming each day. One of the components that you need is your body weight. I brought in a scale for them to weigh themselves on. They were to take their body weight, divide it by 2 and then multiply by 0.05 to attain the amount of protein they need daily in ounces. Since most nutrition fact labels measure protein in grams, I gave them the conversion. 1 oz = 28 grams So if you needed 1.5 ounces of protein a day, that would equal 44 grams of protein |
We finished with our fat note taking guide and talked about the three kinds of fat, saturated, unsaturated and trans fat. As mentioned above, we finished our class documenting the results of our fat test that we started at the beginning of class.
If you would like to know what our note taking guides, take a took at our TpT page.
If you would like to know what our note taking guides, take a took at our TpT page.
Week 9: Carbohydrates
We began class today with our Building Towers Cooperative Activity.
During this activity, I ask review questions and whoever answers the question correctly, will receive a building block. When I am done asking questions, they can use the blocks to build the highest tower they can.
Usually one student would have 2 or 3 blocks which by themselves cannot build a very massive tower, however, if they collaborate and share their blocks with others in the class who have also earned blocks, they can build a much higher tower.
This is hard. This is hard for elementary students, middle school students, high school, college, and adults. We want to do everything on our own, we don't want to work together, we don't want to share and we definitely want to do everything OUR way. The science community is no different. I pray that by having this activity, the students can learn to collaborate, share and build each other up.
During this activity, I ask review questions and whoever answers the question correctly, will receive a building block. When I am done asking questions, they can use the blocks to build the highest tower they can.
Usually one student would have 2 or 3 blocks which by themselves cannot build a very massive tower, however, if they collaborate and share their blocks with others in the class who have also earned blocks, they can build a much higher tower.
This is hard. This is hard for elementary students, middle school students, high school, college, and adults. We want to do everything on our own, we don't want to work together, we don't want to share and we definitely want to do everything OUR way. The science community is no different. I pray that by having this activity, the students can learn to collaborate, share and build each other up.
And finally, they came together. Some had to give up their way of building AND their earned blocks so that the group collectively could build a higher tower. May the Lord be praised for the sacrifices we make for Him.
Our review led right into our next topic of study which was Carbohydrates. Since we began the semester with a foundation in chemistry, I placed the word 'Carbohydrate' on the board and asked them what elements/atoms were contained in a carbohydrate.
They immediately saw the word 'hydrate' and deduced that water, or at least the atoms that make up water would be contained in a carbohydrate. From the word 'hydrate' we figured out that there was hydrogen atoms and oxygen atoms within carbohydrates.
Then we looked at 'Carbo' and another student deduced that it could mean 'Carbon'.
Exactly!!!
There are Carbon, Hydrogen and Oxygen atoms combined to make carbohydrates! This is getting exciting!
They immediately saw the word 'hydrate' and deduced that water, or at least the atoms that make up water would be contained in a carbohydrate. From the word 'hydrate' we figured out that there was hydrogen atoms and oxygen atoms within carbohydrates.
Then we looked at 'Carbo' and another student deduced that it could mean 'Carbon'.
Exactly!!!
There are Carbon, Hydrogen and Oxygen atoms combined to make carbohydrates! This is getting exciting!
I used this note taking sheet to inform the students about what carbohydrates are and what they do for our bodies. They are an important macro nutrient and should not be completely eliminated from our diets.
The topics I covered in this note taking guide included:
I reminded the students of when Jesus told us in Matthew 4:4 that man does not live on bread alone (a carbohydrate) but on every word that comes from the mouth of God. He was quoting Deuteronomy 8:3.
I challenged them to read and study their Bible as much as they eat. Oh, how we might know and walk in God's ways if we feasted on God's Word more!
The topics I covered in this note taking guide included:
- Simple and Complex Carbohydrates
- Blood Sugar Level
- Glycemic Index
- How our body breaks it all down and stores energy.
I reminded the students of when Jesus told us in Matthew 4:4 that man does not live on bread alone (a carbohydrate) but on every word that comes from the mouth of God. He was quoting Deuteronomy 8:3.
I challenged them to read and study their Bible as much as they eat. Oh, how we might know and walk in God's ways if we feasted on God's Word more!

For the carbohydrate on top (cherry on top) we did a Starch Test Demonstration.
I explained that scientists have found that iodine chemically reacts with starches. Starches are a form of complex carbohydrate that is found in some foods. Our task was to figure out if starch is in every carbohydrate or just certain ones. Starch is actually a sugar that plants make and can store.
The foods that I tested were:
Paper towel (This was for a baseline so the students could see what color the iodine would be with no starch in a food)
Cheese
Cracker
Potato
Sweet Potato
Table sugar
Apple Slice (I actually forgot to bring an apple this day but it is included on the experiment sheet.)
I did not have the students handle the iodine as some students might have allergic reactions. It was not worth the risk for me.
The students made their predictions and then I dropped iodine on the foods. If there is starch in the food, the reaction does take about a minute to happen.
In the below pictures, the left picture was just after I finished putting all the iodine on the foods. The picture on the right was after about an hour of the iodine being on the food.
It does appear that the table sugar has starch in it from the picture, but we documented in our observations that it did not change into a dark blue color.
It was interesting how long it took the sweet potato to turn. You can see the difference in the cracker and regular potato but it took a little while for the color change to show up on the sweet potato.
I hope that you will enjoy this demonstration as much as we did!
I explained that scientists have found that iodine chemically reacts with starches. Starches are a form of complex carbohydrate that is found in some foods. Our task was to figure out if starch is in every carbohydrate or just certain ones. Starch is actually a sugar that plants make and can store.
The foods that I tested were:
Paper towel (This was for a baseline so the students could see what color the iodine would be with no starch in a food)
Cheese
Cracker
Potato
Sweet Potato
Table sugar
Apple Slice (I actually forgot to bring an apple this day but it is included on the experiment sheet.)
I did not have the students handle the iodine as some students might have allergic reactions. It was not worth the risk for me.
The students made their predictions and then I dropped iodine on the foods. If there is starch in the food, the reaction does take about a minute to happen.
In the below pictures, the left picture was just after I finished putting all the iodine on the foods. The picture on the right was after about an hour of the iodine being on the food.
It does appear that the table sugar has starch in it from the picture, but we documented in our observations that it did not change into a dark blue color.
It was interesting how long it took the sweet potato to turn. You can see the difference in the cracker and regular potato but it took a little while for the color change to show up on the sweet potato.
I hope that you will enjoy this demonstration as much as we did!
Week 8: Mixtures and Solutions
|
Bonus Week for Science!!
I was so happy to bring my students into my kitchen this week for a lesson on Mixtures and Solutions!! At the beginning we learned some fundamentals of what mixtures and solutions are, some common characteristics and examples of each. We were able to go over the notes provided here. As I was formulating this lesson, the scripture that came to mind is how in the last days, the angels will separate the sheep from the goats. As the Bible says, God has planted good seed in our world but Satan has planted bad seed right alongside the good seed. Instead of God having the angels pull out all the weeds, which would also uproot His good seed, He is waiting until harvest to separate the good from the bad. Matthew 25:32 "Before him will be gathered all the nations, and he will separate people one from another as a shepherd separates the sheep from the goats." In essence, we, on earth, are mixture of good and bad of which only God can separate. |
Separating a mixture
I began with the mixture of Orange Juice and separating the pulp from the juice. I used Pulp Orange Juice, a glass, a funnel and a coffee filter. This took an amazing long time and so we ended up using a fine mesh colander to separate this mixture.
Creating a mixture
Our next activity was to create a mixture. I had the students make a homemade duck feed since we would be going out to the pond a little later with their younger siblings to learn about pond science.
As we were making the duck feed, I would ask them if this would be a good example of a mixture.
Are there two or more pure substances?
Does each substance in the mixture maintain its same properties?
Can the different substances be physically separated?
Are there two or more pure substances?
Does each substance in the mixture maintain its same properties?
Can the different substances be physically separated?
If you would like to make your own duck feed mixture. Here is the recipe:
2 cups Old Fashioned Oats
1 cup brown rice
1 cup of mixed veggies
2 cups Old Fashioned Oats
1 cup brown rice
1 cup of mixed veggies
making a solution
A solution is a type of mixture where one substance is dissolved into another. Pulling in information that we learned last week about solvents and solutes, we talked about how a solute is dissolved in a solvent. We remembered how water is an Amazing Solvent because so many other substances can be dissolved in it.
We made a solution of sugar water today for a hummingbird feeder.
You would take 2 cups of filtered water and dissolve 1/2 cup sugar in the water. I poured the ingredients into the pan and had the students stir the sugar in as the water heated up.
We then discussed what was happening to the water molecules as they were warming up. They were becoming more active allowing the sugar to be broken down into smaller parts and dispersed within the water. The water is the solvent and part of a solvent's job is to hold back the solute and keep it separated thus making our sugar water solution.
We made a solution of sugar water today for a hummingbird feeder.
You would take 2 cups of filtered water and dissolve 1/2 cup sugar in the water. I poured the ingredients into the pan and had the students stir the sugar in as the water heated up.
We then discussed what was happening to the water molecules as they were warming up. They were becoming more active allowing the sugar to be broken down into smaller parts and dispersed within the water. The water is the solvent and part of a solvent's job is to hold back the solute and keep it separated thus making our sugar water solution.
Making Butter
Our final venture was separating some parts of milk.
Milk is a common mixture that has
87% water,
5% lactose,
4% fat,
3% protein and
1% ash
Most of the milk that you would buy at the grocery story is homogenized, meaning that it has been shaken so much that the fat molecules have been broken down and dispersed throughout the milk instead of having the fat molecules clump and rise to the top of the milk.
However, cream from non-homogenized milk is used to make butter, cheese, ice cream, and whipped cream.
I went ahead and bought some heavy whipping cream at the grocery store. Our goal today was to turn that cream into butter.
What is butter? Vigorously shaken cream which makes fat molecules clump together
What is the liquid left behind called? Buttermilk
What is whip cream? a liquid with air trapped inside to make it a foam.
I placed the students into groups of 2-3 so that when they were tired of shaking, they could pass it off to a partner to continue the vigorous shaking.
I filled a half-pint mason jar half full with heavy whipping cream and put the top on.
The students then shook the cream for about 7 minutes. That is a long time to shake something vigorously. They would shake and shake, and we would check and it would still be whip cream.
Finally, the liquid separates from the solid fat and you have butter.
Once the butter is formed, you will drain the buttermilk and keep for pancakes later, then rinse the butter under cold water until all the buttermilk has been rinsed off. Form into butter or place in a butter mold. I formed a ball and placed each of the student's butter in a plastic bag to put in my fridge.
It was a success!
Milk is a common mixture that has
87% water,
5% lactose,
4% fat,
3% protein and
1% ash
Most of the milk that you would buy at the grocery story is homogenized, meaning that it has been shaken so much that the fat molecules have been broken down and dispersed throughout the milk instead of having the fat molecules clump and rise to the top of the milk.
However, cream from non-homogenized milk is used to make butter, cheese, ice cream, and whipped cream.
I went ahead and bought some heavy whipping cream at the grocery store. Our goal today was to turn that cream into butter.
What is butter? Vigorously shaken cream which makes fat molecules clump together
What is the liquid left behind called? Buttermilk
What is whip cream? a liquid with air trapped inside to make it a foam.
I placed the students into groups of 2-3 so that when they were tired of shaking, they could pass it off to a partner to continue the vigorous shaking.
I filled a half-pint mason jar half full with heavy whipping cream and put the top on.
The students then shook the cream for about 7 minutes. That is a long time to shake something vigorously. They would shake and shake, and we would check and it would still be whip cream.
Finally, the liquid separates from the solid fat and you have butter.
Once the butter is formed, you will drain the buttermilk and keep for pancakes later, then rinse the butter under cold water until all the buttermilk has been rinsed off. Form into butter or place in a butter mold. I formed a ball and placed each of the student's butter in a plastic bag to put in my fridge.
It was a success!
Here are some of my class as we rolled into the park for pond science with their younger siblings. Check it out under Creation Science and The World in His Hands Part 2
Week 7: The Wonder of Water

We began our time of instruction with a devotion from Louie Giglio's Indescribable called "Water, Water Everywhere." This highlights how God provided for us so adequately through His creation of water. Its magnificent!
John 4:10
Jesus answered her, "If you knew the gift of God, and who it is that is saying to you, 'Give me a drink,' you would have asked him , and he would have given you living water."
Water is to Physical Life as Salvation through Jesus is to eternal life.
John 4:10
Jesus answered her, "If you knew the gift of God, and who it is that is saying to you, 'Give me a drink,' you would have asked him , and he would have given you living water."
Water is to Physical Life as Salvation through Jesus is to eternal life.

And so we began learning about all the wonders of water. I used this note-taking guide to help us along. As we read the information, the students have blanks to fill in with a word bank. I find this to be a great way for the students to interact and participate in the learning process. Those that do not like to write, can just write the letter than correlates to the correct term and then they call out the letter and I write it next to the term as I write each word that goes in the blanks on the board.
The last time that I taught about water in a chemistry class, water was termed the Universal Solvent. It has now been referenced as the 'almost' Universal Solvent. I think that water is amazing and so I have deemed water as the Amazing Solvent.
We talked about how water makes up 83% of our blood and our blood is what carries nutrients and oxygen to the rest our body. The water in our blood can dissolve those nutrients in order to carry them to where they need to go. I think this is amazing!
Finally, we talked about several different properties of water; cohesion, specific heat capacity and high boiling point. I was able to demonstrate specific heat capacity and the students were able to test water's cohesion by testing its surface tension.
The last time that I taught about water in a chemistry class, water was termed the Universal Solvent. It has now been referenced as the 'almost' Universal Solvent. I think that water is amazing and so I have deemed water as the Amazing Solvent.
We talked about how water makes up 83% of our blood and our blood is what carries nutrients and oxygen to the rest our body. The water in our blood can dissolve those nutrients in order to carry them to where they need to go. I think this is amazing!
Finally, we talked about several different properties of water; cohesion, specific heat capacity and high boiling point. I was able to demonstrate specific heat capacity and the students were able to test water's cohesion by testing its surface tension.

Specific Heat Capacity Demonstration
Materials:
Next, I poured some water in a different plastic bag. I held this water above the open flame and nothing happened. The bag didn't burn up and the water didn't really heat up and yet it was directly on an open flame. Each student was able to feel the bag as it came off the flame.
Explanation: Because water has a high specific heat capacity, it is able to absorb a lot more heat than most other substances. This also contributes to another property of water which is a high boiling point. This is important since the sun is a very hot star and so without all the water on earth to absorb a good amount of the sun's heat, we would be much much hotter.
Materials:
- Plastic bag (2)
- Water
- Candle
- Lighter
Next, I poured some water in a different plastic bag. I held this water above the open flame and nothing happened. The bag didn't burn up and the water didn't really heat up and yet it was directly on an open flame. Each student was able to feel the bag as it came off the flame.
Explanation: Because water has a high specific heat capacity, it is able to absorb a lot more heat than most other substances. This also contributes to another property of water which is a high boiling point. This is important since the sun is a very hot star and so without all the water on earth to absorb a good amount of the sun's heat, we would be much much hotter.

In order to test the surface tension of water, we did this penny experiment. All you would need is some water, a penny, an eye dropper, a paper cup and a paper towel. Each student had to predict how many drops of water a penny could hold. Then they had to run the test three times. They were also supposed to find the average of their trials and place it in their analysis in order to complete the conclusion. I love how this experiment sheet sets up the steps to walk through the scientific method.
Week 6: NUTRIENTS AND CALORIES
We began today's class with our Superhero Element Presentations. The idea behind doing this activity was to allow the students some familiarity with some of the elements of the periodic table. Did some get distracted by all the super hero ideas? Yes. However, for some, we learned a whole lot more about certain elements.

This week, I merged the study of chemistry with some nutrition. The next activity we did was take notes on the Nutrients and Calories Note- taking sheet.
This note-taking sheet defines a calorie in chemistry terms as well as food terms. It also talks about the 11 elements that can be found in our bodies all the while reviewing what atoms, molecules and elements are. The note taking guide introduces six main nutrients that our body needs to be healthy and addresses Jesus' words from Matthew 4:4 that states that man does not live on bread alone but on every word that comes from the mouth of God.
I asked the students if they read their Bible as much as they ate? Hmmm. If we do not receive enough of the Bible in our lives then our spiritual life is not healthy in the same way not getting enough nutrients for our bodies makes our physical bodies unhealthy.
This note-taking sheet defines a calorie in chemistry terms as well as food terms. It also talks about the 11 elements that can be found in our bodies all the while reviewing what atoms, molecules and elements are. The note taking guide introduces six main nutrients that our body needs to be healthy and addresses Jesus' words from Matthew 4:4 that states that man does not live on bread alone but on every word that comes from the mouth of God.
I asked the students if they read their Bible as much as they ate? Hmmm. If we do not receive enough of the Bible in our lives then our spiritual life is not healthy in the same way not getting enough nutrients for our bodies makes our physical bodies unhealthy.
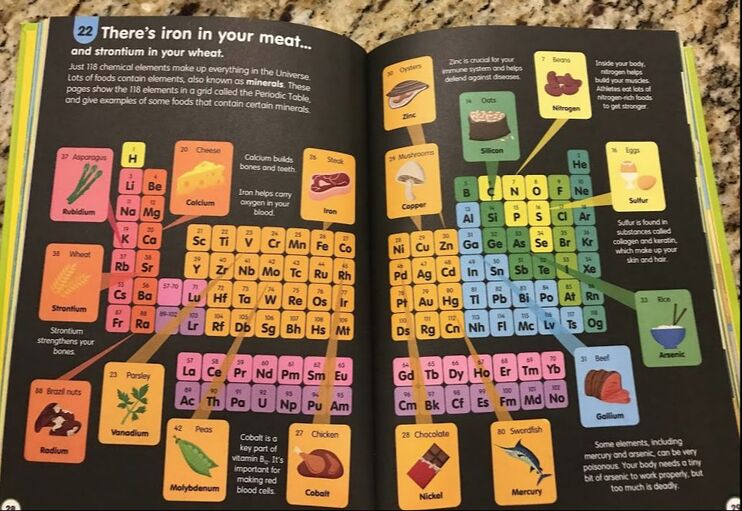
Launching off of the note-taking guide, we then went on a Periodic Table Scavenger Hunt to find the 11 elements that can be found in our bodies. The Periodic Table Scavenger Hunt gave the students the name of the element. They were to find the atomic number, the atomic weight and the abbreviation.
The next step on the scavenger hunt was to then draw the atom with the correct number of protons, neutrons and electrons.
Some information to know before your students conquer this are:
1. Reviewing that the atomic number is the number of protons and electrons in an atom.
2. Teaching that in order to find the number of neutrons, you would subtract the atomic number from the atomic weight.
3. Review that there are a certain number of electrons that fit on certain energy levels. There is a diagram of this on the Scavenger Hunt sheet for their reference
This was a great exercise to apply all the chemistry information that we have been learning these past few weeks and adding in some nutrition as we venture into nutrition and food science.
The next step on the scavenger hunt was to then draw the atom with the correct number of protons, neutrons and electrons.
Some information to know before your students conquer this are:
1. Reviewing that the atomic number is the number of protons and electrons in an atom.
2. Teaching that in order to find the number of neutrons, you would subtract the atomic number from the atomic weight.
3. Review that there are a certain number of electrons that fit on certain energy levels. There is a diagram of this on the Scavenger Hunt sheet for their reference
This was a great exercise to apply all the chemistry information that we have been learning these past few weeks and adding in some nutrition as we venture into nutrition and food science.
For the finale, I had them identify atomic cookies before leaving and they left with a sweet treat. One of the pieces of information that I mentioned in the note-taking guide is that a cookie has more energy in it than a stick of dynamite. If that being so, why don't we explode when we eat it? The answer to this is in the note-taking guide. At the end of our discussion, we praised the Lord that we can enjoy a cookie here and there and not explode. God is good.
These were incredibly easy to make since I used the ready made sugar cookie dough that was on sale for a dollar! I did splurge on the ready made icing that comes with a nozzle to draw with and then the mini M&Ms. If you are going to make regular sized (about 3 inch diameter) cookies, the mini M&Ms are the way to go! The red are the protons, the yellow are the neutrons, and the blue are the electrons.

The students homework for this week was to be aware of the nutrition labels of the food they eat and see if they can document how many calories/energy they are taking in with some of their foods. I will see how they do on this next week, but here is the handout at our TpT store.
|
We are experiencing uncharacteristic ice and snow in central Texas and so today is a snow day which constitutes this week of class to be done online.
Even with the uncertainty of the weather, God is still in control. Let us begin with our Bible verse: "God is not the God of confusion but of order and peace." 1 Corinthians 14:33 We will be learning about how God has so ordered our natural world and how and who figured out how He ordered it. The first task for students to complete is to fill in the note taking guide to familiarize themselves with the information that we will cover today.
I do have a video on YouTube that goes through this guide as I would have done during class. |
The second task that students are to complete is putting together the Periodic People. You can find this resource at
here
here
Here are pictures of the periodic people mixed up and then sorted for your reference.
After completing the Periodic People activity and answering questions 1-3. Watch the TedED talk on Dmitri Mendeleev on YouTube

In the coming weeks, we will take our chemistry knowledge and apply it to Food science and Nutrition and so I showed this picture on my final video for the students as a bridge into what we will be studying for the rest of the semester.
Week 4: Atoms and Molecules
|
John 1:3-"All things were made through Him. Nothing was made without Him."
Indescribable by Louie Giglio We began science class with this devotion 'Atoms, Electrons, Quarks, and Stuff'. The activity I used to introduce atoms and molecules to the students was having them manipulate tangrams into different images. There were some rules to follow: 1. All seven pieces had to be used. 2. Each piece had to lie flat. 3. Each piece must touch at least one other piece. 4. Pieces may not overlap. 5. Pieces CAN be flipped and rotated. As they were making their creations, I told them that these pieces are like atoms. There are a set number of them. They can be manipulated in multiple ways to make different creations but they have to follow the rules laid out by their creator. Just as the Lord God has set before us rules to follow in the 10 Commandments, rules that are for our good and His Glory, He has also made natural laws to govern His creation as well. This is the way His creation works the best. Typically, with any toy, device, appliance that you might purchase, it comes with a set of guidelines or rules to follow in order to maintain your device or appliance best. Our Creator God has done the same thing with His Creation. |

After manipulating the tangrams, we dove right into learning about atoms with our note-taking guide. These guides are so helpful to me as they activate any prior knowledge that the students have had but also present information in an interactive way. Each guide has a word bank of possible choices. This narrows the choice of words down and gives students success. The positive feedback is a great start to motivate more learning. You can purchase these guides from our TpT sight for almost nothing.
One of the concepts that we learned about atoms was that they were incredibly small. That you couldn't even see atoms with a normal microscope.
The question posed was that if the atoms were so small and no one could see them, how do we know they exist? We learned that scientists were able to see how certain atoms behaved and how they can combine with other atoms.
To demonstrate this concept, I gave them a ball of playdoh with an object imbedded and a toothpick. I told them to poke around in the playdoh, not tearing it apart but just poking. As they are poking they were to draw what they were observing as they were poking and thus make some predictions as to what it could be. This is similar to how scientists learned about atoms. They had to poke around matter, take lots of notes about what they were observing and then formulate hypothesis to test their ideas.
Once again, the excuse of not believing in a Creator God because you cannot see Him is debunked. We cannot see atoms but we know they are there. We cannot see God however we know He is here because He is.
The question posed was that if the atoms were so small and no one could see them, how do we know they exist? We learned that scientists were able to see how certain atoms behaved and how they can combine with other atoms.
To demonstrate this concept, I gave them a ball of playdoh with an object imbedded and a toothpick. I told them to poke around in the playdoh, not tearing it apart but just poking. As they are poking they were to draw what they were observing as they were poking and thus make some predictions as to what it could be. This is similar to how scientists learned about atoms. They had to poke around matter, take lots of notes about what they were observing and then formulate hypothesis to test their ideas.
Once again, the excuse of not believing in a Creator God because you cannot see Him is debunked. We cannot see atoms but we know they are there. We cannot see God however we know He is here because He is.
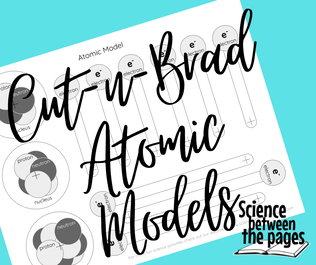
We went from discovering atoms to making models of atoms. This handout was incredibly simple for the students to make. I printed these out on cardstock, and then for ease for the students, I precut slits in the middle so that their brads could be easily inserted through the cardstock. I found this activity to be less cumbersome than other model making activities so that I could focus on teaching about the parts of the atom versus how to put the model together.
I did NOT tell them which atom these were. That was for them to discover by doing their homework this week.
So thankful for a great class!!!
I did NOT tell them which atom these were. That was for them to discover by doing their homework this week.
So thankful for a great class!!!
Week 3: Non-Newtonian Fluids
These are the warm ups from class today.
Name some physical properties of matter.
States of Matter
Which 'change' and which 'stay the same'?
Solid's volume-stays the same
Solid's shape- stays the same
Solid's mass- stays the same
Liquid's volume- changes
Liquid's shape- changes
Liquid's mass- stays the same
Gas' volume- changes
Gas' shape- changes
Gas' mass- stays the same
Name some physical properties of matter.
- texture
- state of matter
- weight
- length
- shape
- temperature
- magnetic
- density
- odor
- mass
- volume
States of Matter
Which 'change' and which 'stay the same'?
Solid's volume-stays the same
Solid's shape- stays the same
Solid's mass- stays the same
Liquid's volume- changes
Liquid's shape- changes
Liquid's mass- stays the same
Gas' volume- changes
Gas' shape- changes
Gas' mass- stays the same

Our investigation today centered on a research question dealing with the states of matter. This investigation was structured in a way that lends itself to doing a science fair project.
Our research question was:
How can I tell what state of matter a mystery substance is?
From this we
The students even went a step further and didn't just discover what the state of matter the mystery substances were but also guessed what substance it was!!
Our research question was:
How can I tell what state of matter a mystery substance is?
From this we
- Gathered some background research
- Formulated a hypothesis
- Went through the procedures
- Gathered Data
- Analyzed the data
- Made our conclusions
The students even went a step further and didn't just discover what the state of matter the mystery substances were but also guessed what substance it was!!

After discovering what our mystery substances were we categorized them into either a Newtonian fluid or a non-Newtonian Fluid.
We learned that Newtonian fluids have a direct connection between their temperature and their viscosity whereas Non-Newtonian Fluids do not follow those same rules. The viscosity of a non-Newtonian fluid is dependent on the amount of pressure applied rather than the temperature.
Some wonderful applications to this information were that the synovial fluid around our knee and elbow joints is actually non-Newtonian. This was so well designed by our Creator. He knew that these joints would need some extra protection. The kind of non-Newtonian fluid that synovial fluid is called shear thickening. This means that when pressure is applied to these joints, the fluid hardens thus protecting the elbow and knee joint. Amazing!
"We are pressed on every side by troubles, but we are not crushed." 2 Corinthians 4:8a
The notes above go into more detail about these fluids.
We learned that Newtonian fluids have a direct connection between their temperature and their viscosity whereas Non-Newtonian Fluids do not follow those same rules. The viscosity of a non-Newtonian fluid is dependent on the amount of pressure applied rather than the temperature.
Some wonderful applications to this information were that the synovial fluid around our knee and elbow joints is actually non-Newtonian. This was so well designed by our Creator. He knew that these joints would need some extra protection. The kind of non-Newtonian fluid that synovial fluid is called shear thickening. This means that when pressure is applied to these joints, the fluid hardens thus protecting the elbow and knee joint. Amazing!
"We are pressed on every side by troubles, but we are not crushed." 2 Corinthians 4:8a
The notes above go into more detail about these fluids.
Week 2:
States of Matter
|
As the students arrived at class, they took out their physical properties homework from last week. They were to calculate the densities of the objects that we found the mass and volume for the previous week and make a prediction from their numbers whether an object would sink or float. I absolutely loved the interaction between math and science in this activity. By doing this activity, the students were able to see a pattern in the numbers as to what would sink and what would float. If the density of an object is equal to or greater than 1g/ml it will sink. If the density of an object is less than 1g/ml then it will float.
We had only one object that did not float as the numbers said that it should. This was perfect! We realized that our numbers for our calculations had been wrong and so going back to correct those would yield the correct density reflecting that the object did indeed sink. |
Digging deeper into the physical properties of matter, we discovered the states of matter and learned about how the molecules are acting in an object or substance.
States of Matter are how tightly packed the molecules of a substance are and how much energy it takes to get the molecules to move apart.
The three states of matter that we talked about during this class were Solids, Liquids, and Gases. In our handout we described the attributes of each state of matter.
Can a substance change states?
Yes.
We discovered that in order for a substance to change states, it requires kinetic energy.
In the midst of talking about changing states of matter, it is good to note that our Almighty God does NOT change. He has created substances and objects to be able to change on earth for His purposes, however He is a God who never changes. James 1:17 states "Whatever is good and perfect is a gift coming down to us from God our Father, who created all the lights in the heavens. He never changes or casts a shifting shadow." (NLT)
States of Matter are how tightly packed the molecules of a substance are and how much energy it takes to get the molecules to move apart.
The three states of matter that we talked about during this class were Solids, Liquids, and Gases. In our handout we described the attributes of each state of matter.
Can a substance change states?
Yes.
We discovered that in order for a substance to change states, it requires kinetic energy.
In the midst of talking about changing states of matter, it is good to note that our Almighty God does NOT change. He has created substances and objects to be able to change on earth for His purposes, however He is a God who never changes. James 1:17 states "Whatever is good and perfect is a gift coming down to us from God our Father, who created all the lights in the heavens. He never changes or casts a shifting shadow." (NLT)
|
The most common agent of phase change from one state of matter to another is heat.
Discovering that melting points are the results of temperature and pressure transforming solid to liquids, we did this melting point demonstration. I placed an ice cube, a paper clip, and a chocolate kiss in three separate containers within a glass pyrex dish. The demonstration was to see how long it would take each solid to attain its melting point. In my electric kettle, I allowed water to reach its boiling point when the temperature and pressure would be enough to change the liquid into a gas. I poured the boiling water into the pyrex dish and started a timer to see how long it would take the solids to melt. The result was that it took the ice cube 4 minutes 17 seconds to completely melt. The chocolate kiss was softened immensely but never lost its shape The paper clip was slightly heated. We determined that the heat produced from the boiling water was not enough heat or pressure to reach the melting point for the paperclip. |
This demonstration was a wonderful activity to do because it allowed us to take some time to use our observational skills and determine what was happening during the 4 minutes we were waiting for the ice to melt. During this time, the students were commenting on different ways we could do the demonstration. One suggestion was to pour the boiling water inside the cups with the object. We talked about the benefits to this procedure and the results it might have. Another student suggested covering the pyrex container with saran wrap to help trap some of the heat in order for the melting points to be reached sooner. This was a wonderful display of thinking and reasoning with each other.

For their homework this week, I am having the students read some more notes that we created about the states of matter that are with the above mentioned note-taking guide. The information in these notes were taken from the Answers in Genesis God's Design Curriculum in the Properties of Matter book.
Also, sometime during the week, they are to watch the video about States of Matter from Study Jams. Here is the link
http://studyjams.scholastic.com/studyjams/jams/science/matter/solids-liquids-gases.htm
Also, sometime during the week, they are to watch the video about States of Matter from Study Jams. Here is the link
http://studyjams.scholastic.com/studyjams/jams/science/matter/solids-liquids-gases.htm
Week 1:
Getting Started with Physical and Chemical Properties
|
This class is called the Manna of Things as we will be discovering some chemistry as it relates to nutrition and food science.
Manna is the word the Israelites used to call the food that God provided for them in the desert. It means, "What is it?" Is not the basic study of chemistry, matter, a question of What is it? And so we begin our adventures into discovering what stuff is really made of. Nehemiah 9:6 "You are the LORD, You alone. You have made heaven, the heaven of heavens with all their host, the earth and all that is on it, the seas and all that is in them; and You preserve all of them; and the host of heaven worships You." No 'matter' what we find, all things are made by our Creator God and so all glory, honor and credit go to Him for what we discover in the coming weeks. Our first task was to gather some knowledge about chemistry and what it is. We were able to breakdown the different components that make up chemistry. Chemistry is the study of matter and matter is anything that has mass and volume. Chemistry studies matter and its 1. composition 2. structure 3. properties and 4. changes. Mass is the amount of matter something has and volume is the amount of space matter occupies. When we divide the mass and volume of an object, then we attain its density. Physical properties of matter can be observed and measured without changing its substance whereas chemical properties of matter describe how matter has the ability to change into new substance with different properties. All of this information I placed in a note-taking guide for my students. |
When we were able to accomplish a little bit of foundation in chemistry, I had the students partner up and select an item from a mystery bag. They were to note on their data collection sheet its color, length, width, height, mass and volume. We then applied the notes and ideas we had learned to the actual process of discovering what each item really was and how to describe it using the physical properties of matter.
We learned that the length, width and height of a sphere will be the same.
We used 1 gram cubes to determine mass on a scale
We used water displacement to find the volume of an object.
My students will then be working this week to figure out the densities of all the objects in order to predict if they think the object will sink or float. We will test their predictions in class next week and see if we can find a pattern in the numbers we calculated for density and whether the object would sink or float.
Another aspect of their data collection sheet is a challenge for them to do at home in practicing the uses of the physical properties of matter. These handouts you can find at our TPT store for almost free.
We learned that the length, width and height of a sphere will be the same.
We used 1 gram cubes to determine mass on a scale
We used water displacement to find the volume of an object.
My students will then be working this week to figure out the densities of all the objects in order to predict if they think the object will sink or float. We will test their predictions in class next week and see if we can find a pattern in the numbers we calculated for density and whether the object would sink or float.
Another aspect of their data collection sheet is a challenge for them to do at home in practicing the uses of the physical properties of matter. These handouts you can find at our TPT store for almost free.
My student's last assignment for homework is to view the Study Jams video on the Physical Properties of Matter and take their online quiz associated with it. The link for this video is
http://studyjams.scholastic.com/studyjams/jams/science/matter/properties-of-matter.htm
http://studyjams.scholastic.com/studyjams/jams/science/matter/properties-of-matter.htm